Unlocking the Benefits of Paid Clinical Trials: What You Need to Know
Participating in a clinical trial can be a rewarding experience, offering access to specialized medical care and the opportunity to contribute to scientific progress.
What’s even more enticing is that some clinical trials offer compensation for your time and effort. In this article, we’ll explore paid clinical trials, why compensation for medical research is offered, and how to find these opportunities that align with your interests and health.
Understanding Paid Clinical Trial Opportunities
Before diving into the world of paid clinical trials, it’s essential to grasp why compensation is provided in some cases. Typically, compensation serves as a token of appreciation for the risks involved in the trial.
Clinical trials involve testing new treatments and therapies that have not yet received approval, so participants play a crucial role in advancing medical science.
Before enrolling in any clinical trial, participants are required to review and sign an informed consent form (ICF). This document outlines the potential benefits, risks, and side effects associated with the study.
It’s important to recognize that participation is voluntary, and individuals can withdraw at any time. However, the ICF serves as a valuable tool for comprehending the potential risks associated with the trial, making informed decisions a priority.
Getting paid for clinical trials: the variability in compensation
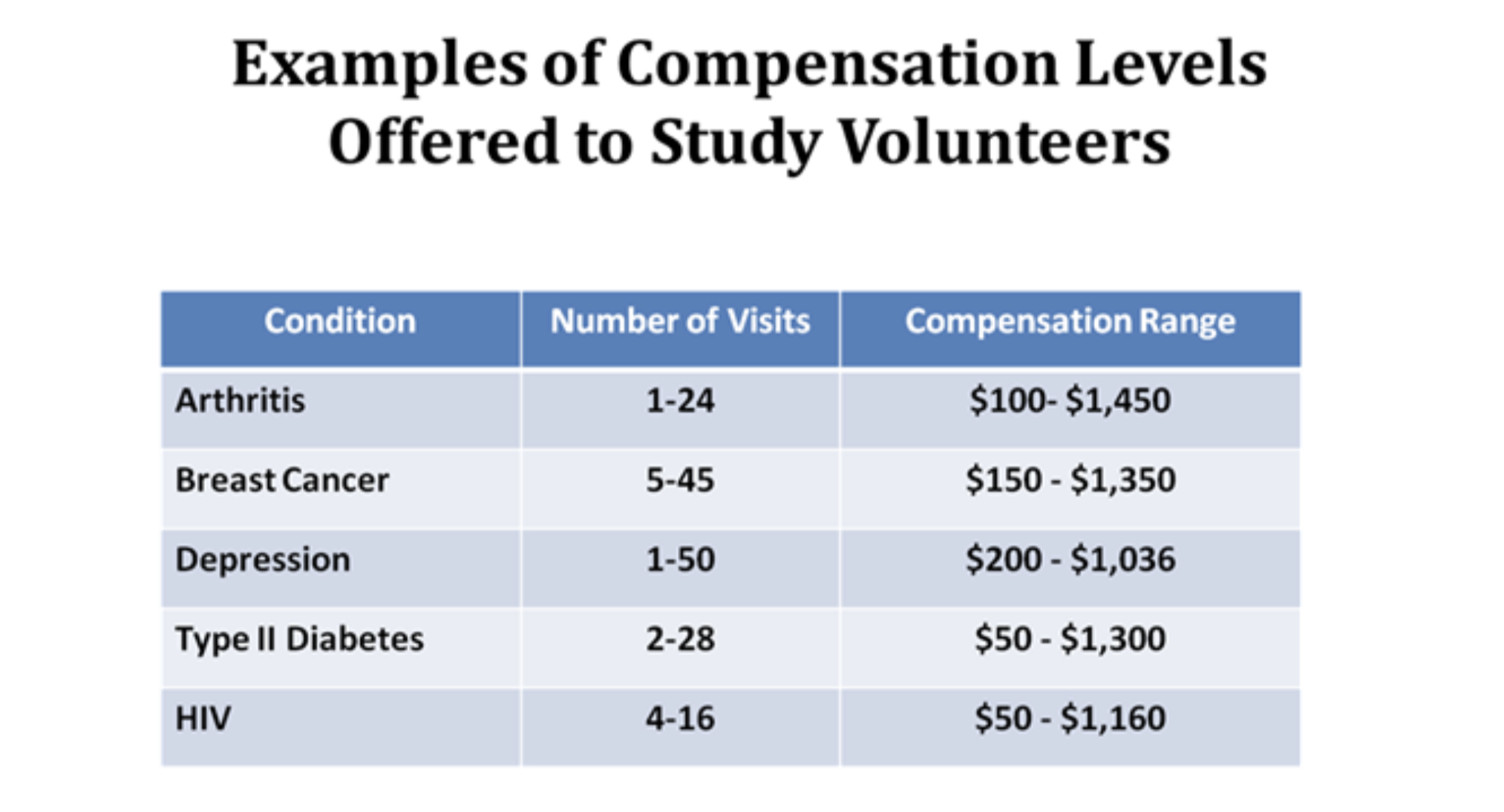
Compensation in clinical trials can vary significantly, depending on various factors. The phase of the trial plays a central role in determining payment rates.
Phase 1 trials, which investigate treatments that are less understood, tend to offer the highest compensation, averaging around $2,000. In contrast, Phase IV trials, focusing on post-approval research, typically offer lower compensation, averaging about $400.
Moreover, the therapeutic area being studied can impact payment rates. Trials related to cardiovascular disease, neurology, endocrine disorders, gastrointestinal conditions, and blood disorders often offer the highest compensation.
However, it’s crucial to remember that compensation is not merely handed out; participants are expected to provide detailed tracking of symptoms and side effects, as well as make periodic visits to the research site.
Clinical trials compensation can vary based on several factors:
- Specialization:
Highly specialized trials may offer higher compensation, particularly if expertise is required from participants. - Duration:
Longer trials tend to offer higher compensation to account for the extended time commitment. - Type of Project:
Trials that involve travel or overnight stays often provide more substantial compensation to cover related expenses. - Complexity:
The complexity of the trial and the level of involvement required can impact compensation rates.
How to Find Paid Clinical Trials

Finding paid clinical trials can be challenging due to strict advertising regulations and eligibility criteria. Many trials do not explicitly mention compensation in their listings, making it difficult to determine eligibility before the screening process. Here’s a guide on how to embark on your quest for paid clinical trial opportunities:
- Use a Personalized Search:
Start by using personalized clinical trial search tools to narrow down options. While these tools may not allow you to filter for paid trials, they help identify studies that align with your profile and needs. - Screening Process:
Keep in mind that most eligibility and compensation details are revealed during the screening process. Once you identify a trial that interests you, undergo the initial screening to determine your eligibility and gain insights into the compensation offered. - Consider the Benefits:
Even if a clinical trial doesn’t provide compensation, remember that you’re contributing to scientific progress and potentially gaining access to innovative treatments. Your participation is invaluable in advancing medical research and benefiting future generations.
Benefits of Participating in Clinical Trials
Joining a clinical trial can be a meaningful decision, offering several advantages:
- Access to Innovative Treatments:
Clinical trials often involve testing new medications or therapies. By participating, you might gain early access to cutting-edge treatments that could improve your health. - Contribution to Medical Advancements:
Your involvement directly contributes to the advancement of medical knowledge. Researchers rely on participants like you to gather crucial data that can lead to better healthcare practices. - Personalized Care:
Clinical trials typically involve close monitoring and personalized care plans, ensuring that your health is a top priority throughout the study. - Financial Compensation:
Many clinical trials offer compensation to participants. This compensation can vary based on the trial’s complexity, duration, and required commitment.
Safety Considerations: Are paid clinical trials safe?
Safety is paramount in paid medical studies. Before participating, it’s essential to understand the potential risks and inconveniences:
- Time Commitment:
Clinical trials can be time-consuming. Participants may need to attend multiple screening and follow-up sessions. Some trials even require overnight stays. - Restrictions:
You may encounter restrictions on daily activities, such as dietary limitations or abstinence from alcohol consumption. - Possible Side Effects:
New treatments can have unknown side effects. Be prepared for the possibility of experiencing unexpected reactions to the study intervention. - Informed Consent:
Researchers are required to obtain your informed consent before your participation. Take the time to thoroughly review the study’s details and ask questions to ensure you understand the risks and benefits.
Conclusion
In the realm of clinical trials, compensation can be a welcome incentive for participants, acknowledging the risks and efforts involved. However, it’s crucial to strike a balance between compensation, contribution to medical research, and safety of paid clinical trials. Research studies are essential for advancing healthcare and developing new treatments, and without willing participants, progress would be significantly hindered.
Before joining a clinical trial, potential participants should carefully review the informed consent form, ask questions, and weigh the potential benefits and risks. Participation in clinical trials is a noble endeavor that can contribute to the well-being of future generations and the advancement of scientific knowledge. Whether compensated or not, every participant plays a vital role in the pursuit of better healthcare solutions. So, if you’re considering joining a clinical trial, start your search today and be part of the progress in medical science.
Tips for Finding and Participating in Paid Clinical Trials from Home in 2024
- Research Reputable Trial Platforms:
Look for established online platforms or organizations that specialize in remote clinical trials. These platforms often list a variety of trials, including those for healthy volunteers. - Set Clear Expectations:
Understand that not all clinical trials offer monetary compensation, especially those focused on treatments for specific medical conditions. If your primary goal is payment, prioritize trials explicitly offering compensation. - Browse Multiple Trial Listings:
Explore different trial databases and listings to find the highest paid clinical trials that align with your profile and interests. Cast a wide net and consider various options. - Healthy Volunteer Trials:
Yoy can specifically search paid clinical trials for healthy volunteers designed for individuals without underlying medical conditions. These trials often offer compensation for your time and participation. - Review Trial Details:
Thoroughly read the descriptions of each trial, paying attention to compensation details, study requirements, and eligibility criteria. Ensure you qualify and are comfortable with the trial’s expectations. - Understand Payment Processes:
Familiarize yourself with how and when you will get paid for clinical trials. Some trials may provide payments at specific milestones, while others may offer a lump sum upon trial completion. - Safety Monitoring:
Paid research studies include regular health assessments and monitoring to detect any adverse effects or complications. Researchers closely track participants’ well-being and can intervene if safety concerns arise.
Your Guide on Paid Research Studies in 2024
Looking to supplement your income while sharing your insights and opinions? Explore the world of paid research studies and discover how participating in paid research can turn your voice into cash.
From online surveys to in-person focus groups, this comprehensive guide will walk you through the different types of paid research studies and the best companies to join for these opportunities.
What Are Paid Research Studies?
Paid research studies, also known as clinical trials, are a fantastic way to earn extra money during your spare time. They’ve become a popular side hustle for many individuals seeking additional income. With numerous studies running simultaneously and a growing number of companies seeking participant expertise, you’re likely to find a a study quickly, especially if you’re new to paid scientific studies.
Understanding Paid Research Studies
A paid research study typically involves an “interview” between you and at least one other person, often qualified medical professionals and researchers. During this interview, you’ll answer a series of questions related to a specific subject, which may or may not be within your area of expertise. The interviewer may work for the research company or represent an organization interested in gathering your insights.
The duration of a paid research study can vary significantly, from a few minutes to an hour or even an entire weekend. Likewise, the compensation you receive depends on the time commitment required for each study.
Different Types of Research Studies
Paid research studies are conducted through various methods, each offering unique advantages and formats:
- Telephone Interviews:
In telephone interviews, you’ll engage in one-on-one conversations with a researcher, sometimes with other listeners. These interviews are typically recorded for analysis. - One-on-One In-Person Meetings:
While less common today due to COVID-19, in-person meetings involve face-to-face interviews conducted either at the researcher’s location or your own. These may be audio or video recorded or have a note-taker present. - Group Meetings/Focus Groups:
Previously conducted in person, focus groups now take place online. Multiple participants meet in a virtual space to discuss a topic or product with a researcher, offering individual and group opinions. - Online Meetings:
The most prevalent method today, paid online research studies involve one-on-one video calls between you and a researcher through platforms like Zoom or Microsoft Teams. - Sampling at Home:
Sampling studies may include testing food, beauty products, or other items at home. You’ll provide feedback on your experiences, either through a one-on-one chat or an online form.
How to Get Paid for Research Studies

One of the most enticing aspects of participating in paid research studies is the opportunity to earn compensation for your time and efforts. While the amount you can earn varies depending on several factors, most research studies offer monetary rewards that can range from $75 to $4,500 or even more. Here’s a breakdown of how compensation is determined:
- Study Requirements:
Compensation is influenced by the study’s specific requirements. This includes the number of in-person visits, phone consultations, symptom tracking, and study-related exams. - Study Type:
The nature of the research study can significantly impact the compensation. Low-paying studies may involve interviews or specimen collection, while high-paying studies often evaluate investigational treatments like vaccines or medications. - Study Phase:Clinical trials are typically conducted in phases, with Phase I being the earliest and Phase IV the most advanced. Phase I studies, which involve more risk and time, tend to offer higher compensation.
- Duration:
The length of the study also plays a role in determining compensation. Longer trials or those with frequent check-ins may offer increased payments.
Taking Part in Compensated Research Studies
In order to participate in paid research studies, you need to register with clinical trials or research companies. Registering typically involves providing basic personal information and answering questions to match you with suitable studies. Once registered, you’ll receive frequent emails listing available studies that match your profile.
By joining multiple companies, you can access a broader range of paid research opportunities and increase your chances of earning money from research studies.
University Paid Research Studies Online
Participating in university-sponsored online research studies can be a rewarding and financially beneficial experience. Universities often conduct research across various fields, and they require participants to provide valuable insights and data. Here’s a concise overview of university paid research studies online:
- Academic Research Opportunities:
Universities frequently engage in academic research studies spanning disciplines such as psychology, economics, sociology, and more. These studies aim to advance knowledge in various fields. - Participation Requirements:
Participants in university research studies typically need to meet specific criteria, such as age, location, or demographic factors. Eligibility criteria vary depending on the study’s objectives. - Online Accessibility:
Many university research studies are now conducted online, making it convenient for participants to take part from the comfort of their homes. Online platforms, surveys, and video conferences facilitate remote participation. - Financial Compensation:
Universities often offer compensation for participants’ time and effort. Payments can vary depending on the study’s complexity, duration, and the level of involvement required. - Diverse Research Topics:
University studies cover a wide range of topics, from cognitive psychology experiments to economic surveys. Participants can choose studies that align with their interests or expertise. - Contributing to Knowledge:
By participating in paid academic research studies, individuals contribute to the advancement of scientific knowledge. Your insights may help researchers make significant discoveries or validate existing theories. - Ethical Considerations:
Universities prioritize ethical research practices, ensuring that participants’ rights and privacy are protected. Before participating, researchers provide informed consent and explain the study’s objectives.
In short, engaging in university paid research studies online offers an opportunity to contribute to academia, expand your understanding of various subjects, and earn compensation for your involvement.
Keep an eye out for research opportunities that match your profile and interests, as they can provide a meaningful and financially rewarding experience.
Tips for Maximizing Your Earnings from Research Studies in 2024
Participating in paid research studies can be a rewarding side hustle, but to make the most of your opportunities, consider these tips:
- Register with Multiple Companies:
Don’t limit yourself to just one paid research company. By registering with several companies, you’ll have access to a broader range of research studies, increasing your chances of finding ones that match your interests and expertise. - Complete Your Profile:
Ensure that your profile with each paid research company is complete and up-to-date. Accurate information about your demographics, interests, and expertise will help companies match you with relevant studies. - Check Your Email Regularly:
Paid research companies often send invitations via email. To secure a spot in high-paying studies, check your email regularly and respond promptly to invitations. - Be Honest:
When participating in research studies, honesty is crucial. Provide genuine responses to questions, as misleading information can disqualify you from future studies and harm your reputation. - Diversify Your Participation:
Explore various types of paid research studies, from online surveys to focus groups and in-person interviews. Diversifying your participation can lead to a more consistent stream of income. - Set Realistic Expectations:
While some specialized studies offer generous compensation, not all of them pay equally. Set realistic expectations for earnings and consider each research study’s time commitment and complexity. - Plan Your Schedule:
Before committing to a research study, assess whether you have the time to participate fully. Avoid overloading your schedule, as this can lead to stress and reduced earnings. - Stay Informed:
Keep up with industry trends and paid research news. Being informed can help you identify lucrative opportunities and stay ahead in the field. - Refer Friends and Family:
Some paid research companies offer referral programs. Encourage friends and family to join, and you may receive bonuses or incentives for successful referrals. - Track Your Earnings:
Maintain a record of your earnings from each study and any applicable tax obligations. Staying organized ensures you can manage your finances effectively. - Protect Your Privacy:
Be cautious when sharing personal information. Legitimate paid research companies prioritize participant privacy, but it’s essential to verify the credibility of any organization you engage with.
By following these tips, you can navigate the world of paid research studies effectively and make the most of your opportunities to earn extra cash.
Conclusion
Participating in paid research studies is a convenient way to earn extra money while sharing your insights and opinions. Whether you prefer online surveys or in-person focus groups, there are numerous paid research studies available.
Follow the steps outlined in this guide, explore different research study types, and register with reputable paid research companies to start turning your voice into cash today.
A Guide to Participation in Clinical Trials: Everything You Need to Know
Introduction
Clinical trials are crucial for advancing medical knowledge and improving healthcare. They offer opportunities for individuals to contribute to groundbreaking research while potentially benefiting from new treatments or earning compensation for their participation.
In this article, we will explore the various aspects of clinical trial participation, including how to get involved, the reasons people choose to participate, questions to ask before joining a trial, and the ethical considerations involved.
What Are Clinical Trials?
Clinical trials are research studies in which volunteers play a vital role in answering specific health-related questions.
These trials follow carefully designed protocols that outline the patient criteria, testing procedures, drug details, dosages, study duration, and research objectives. They are conducted under strict regulations set by the FDA to ensure participant safety.
Participation in Clinical Trials
Clinical trials adhere to a predefined plan known as a protocol. This protocol outlines several key elements:
- Eligibility criteria:
Defines the types of patients who may enter the study. These criteria consider factors such as age, sex, type and stage of disease, previous treatment history, and other medical conditions to ensure study consistency. - Test and procedure schedules:
Outlines the timing and frequency of medical tests and procedures participants will undergo. - Drug information:
Describes the drugs involved in the trial, including their dosages and administration methods. - Study duration:
Specifies the length of time participants will be involved in the trial. - Research objectives:
Details what the researchers aim to learn from the study.
Participants are required to consent to the rules and terms outlined in the protocol. Similarly, researchers, physicians, and healthcare professionals overseeing the clinical trials must adhere to stringent guidelines set by regulatory bodies like the FDA. These rules are designed to ensure the safety of those who volunteer.
Why Participate in Clinical Trials in 2024?
Clinical trials serve several essential purposes:
- Testing New Treatments:
Clinical trials assess the safety and effectiveness of new drugs or medical devices, potentially leading to improved healthcare options. - Enhancing Current Treatments:
They explore innovative ways to use existing treatments, making them more effective or reducing side effects. - Expanding Treatment Options:
Clinical trials aim to identify treatments suitable for specific populations, including children and those who have not responded to standard therapies. - Advancing medical knowledge:
Participation contributes to the progression of medical understanding and potential breakthroughs in healthcare. - Advancing Health Equity:
Ensuring diverse participation in clinical trials is crucial to represent the broader population accurately. People from various backgrounds may react differently to treatments, emphasizing the importance of diversity in research.
Eligibility Criteria and Selection
Clinical trials have eligibility criteria based on factors like age, sex, disease type, previous treatments, and overall health. Not everyone who applies will be accepted, as these criteria aim to reduce variability within the study group.
Finding Clinical Trials
Clinical trials can be sponsored by pharmaceutical companies, federal agencies like the NIH, or individuals such as doctors. They are typically conducted at universities, medical centers, clinics, hospitals, and research sites. Or you can find a list of active clinical trials on our website simply by clicking the “Live Studies” button.
Ensuring Safety in Clinical Trials
Clinical trial participants’ safety is paramount, and rigorous regulations are in place to protect them. Participants receive complete and accurate information about the trial before signing an informed consent document. This document outlines their rights and the study’s details, including potential risks.
Questions to Ask Before Participating in a Clinical Trial
Before participating in a clinical trial, individuals should gather as much information as possible. Key questions to consider include:
- Purpose of the Study:
What is the main objective of this trial? - Study Duration and Procedures:
How long will the trial last, and what will be required of participants? - Placebo Use:
Does the study involve a placebo, and will my doctor know which treatment I receive? - Intervention Details:
How will the research intervention be administered? - Published Results:
What information is available about the research intervention’s results? - Costs and Insurance:
Do I have to pay for any part of the study, and will my insurance cover these expenses? - Travel and Childcare:
Does the study cover travel costs or childcare expenses? - Continuity of Care:
Can I continue using the treatment if it proves effective after the study? - Follow-Up Care:
Will I receive follow-up care after the study concludes? - Research Team:
How experienced are the physician and study staff? - Safety Measures:
What measures are in place to ensure my safety? - Results Disclosure:
When and how will I receive the results of the clinical trial? - Placebo Disclosure:
Will I be informed if I receive a placebo?
- Benefits and risks:
Weigh the potential advantages and disadvantages of joining the trial. Assess whether the benefits align with your health goals. - Informed consent:
Make sure you receive complete and accurate information about the study, including potential risks, and that you sign an informed consent document acknowledging your understanding and the voluntary nature of your participation.
Ethical Considerations
Participating in a clinical trial is a personal choice that should be made voluntarily and without external pressure. Informed consent is a fundamental aspect of clinical research, ensuring that participants understand and agree to the study’s terms. Participants are free to withdraw from a trial at any time.
Enrolling in Clinical Trials
It’s essential to find clinical trials that match your needs and interests. You can start by asking your healthcare provider for recommendations. Additionally, you can explore resources such as the FDA Clinical Trials Search, Clinicaltrials.gov, the National Cancer Institute, and AIDS Clinical Trials and Information Services to find relevant trials.
Participate in Clinical Trials for Money
While some clinical trials offer compensation for participation, it’s important to prioritize your health and align your motivations with the broader goals of advancing medical science. Monetary incentives should not be the sole reason for participating.
Benefits of Participating in Clinical Trials
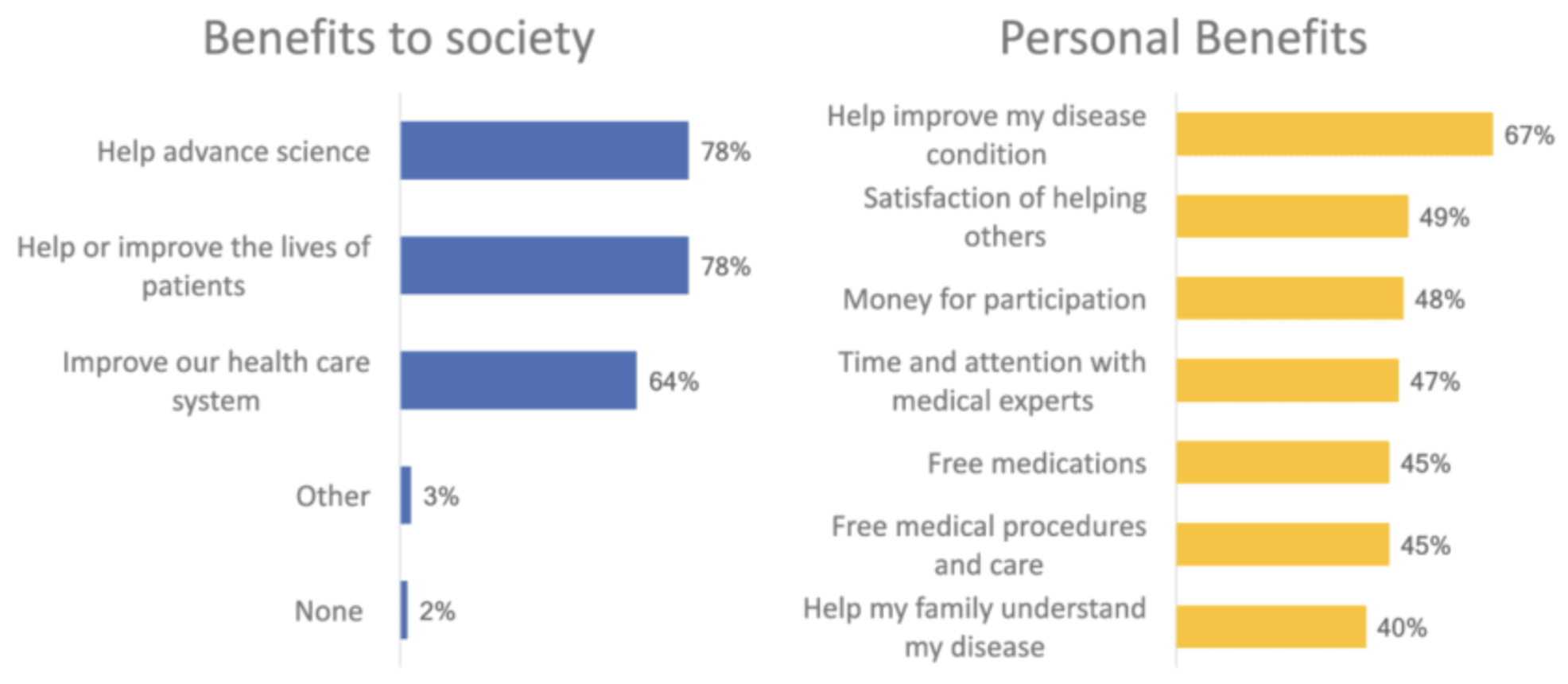
Participating in clinical trials can have various benefits, including access to cutting-edge treatments, expert medical care, and the opportunity to contribute to medical advancements that benefit society as a whole.
Tips on how to participate in clinical trials in 2024

- Do Your Homework:
Research the clinical trial thoroughly. Understand its purpose, procedures, potential risks, and benefits before making a decision. - Ask Questions:
Don’t hesitate to ask the research team any questions or concerns you have about the trial. It’s crucial to have a clear understanding. - Consult Your Healthcare Provider:
Discuss the clinical trial with your regular doctor to ensure it aligns with your current treatment plan and health goals. - Read the Informed Consent Carefully:
Take your time to read and understand the informed consent document. This document outlines your rights and the trial’s details. - Consider the Ethical Aspect:
Reflect on your motivation for participating in the trial. Ensure your decision aligns with your values and is made voluntarily. - Keep a Journal:
Maintain a journal to record your experiences and any changes in your health during the trial. This can be helpful for both you and the research team. - Follow Instructions:
Adhere to the trial’s protocols and follow all instructions provided by the research team. This ensures accurate data collection. - Report Adverse Events:
If you experience any adverse effects or discomfort during the trial, promptly inform the research team. Your well-being is their priority. - Stay Committed:
If you decide to participate, commit to the trial’s duration and requirements. Your contribution is essential for meaningful results. - Share Your Experience:
After the trial, consider sharing your experience with others. Your insights can help prospective participants make informed decisions about clinical trial participation.
Conclusion
Clinical trials are essential for advancing medical knowledge and improving healthcare. By participating in these trials, individuals can contribute to groundbreaking research, potentially benefit from innovative treatments, and even receive compensation for their involvement.
To make an informed decision about joining a clinical trial, it’s crucial to ask questions, consider the ethical aspects, and ensure that your participation aligns with your goals and values. Clinical research not only benefits individuals but also contributes to the betterment of healthcare for future generations.