Introduction
Clinical trials are crucial for advancing medical knowledge and improving healthcare. They offer opportunities for individuals to contribute to groundbreaking research while potentially benefiting from new treatments or earning compensation for their participation.
In this article, we will explore the various aspects of clinical trial participation, including how to get involved, the reasons people choose to participate, questions to ask before joining a trial, and the ethical considerations involved.
What Are Clinical Trials?
Clinical trials are research studies in which volunteers play a vital role in answering specific health-related questions.
These trials follow carefully designed protocols that outline the patient criteria, testing procedures, drug details, dosages, study duration, and research objectives. They are conducted under strict regulations set by the FDA to ensure participant safety.
Participation in Clinical Trials
Clinical trials adhere to a predefined plan known as a protocol. This protocol outlines several key elements:
- Eligibility criteria:
Defines the types of patients who may enter the study. These criteria consider factors such as age, sex, type and stage of disease, previous treatment history, and other medical conditions to ensure study consistency. - Test and procedure schedules:
Outlines the timing and frequency of medical tests and procedures participants will undergo. - Drug information:
Describes the drugs involved in the trial, including their dosages and administration methods. - Study duration:
Specifies the length of time participants will be involved in the trial. - Research objectives:
Details what the researchers aim to learn from the study.
Participants are required to consent to the rules and terms outlined in the protocol. Similarly, researchers, physicians, and healthcare professionals overseeing the clinical trials must adhere to stringent guidelines set by regulatory bodies like the FDA. These rules are designed to ensure the safety of those who volunteer.
Why Participate in Clinical Trials in 2024?
Clinical trials serve several essential purposes:
- Testing New Treatments:
Clinical trials assess the safety and effectiveness of new drugs or medical devices, potentially leading to improved healthcare options. - Enhancing Current Treatments:
They explore innovative ways to use existing treatments, making them more effective or reducing side effects. - Expanding Treatment Options:
Clinical trials aim to identify treatments suitable for specific populations, including children and those who have not responded to standard therapies. - Advancing medical knowledge:
Participation contributes to the progression of medical understanding and potential breakthroughs in healthcare. - Advancing Health Equity:
Ensuring diverse participation in clinical trials is crucial to represent the broader population accurately. People from various backgrounds may react differently to treatments, emphasizing the importance of diversity in research.
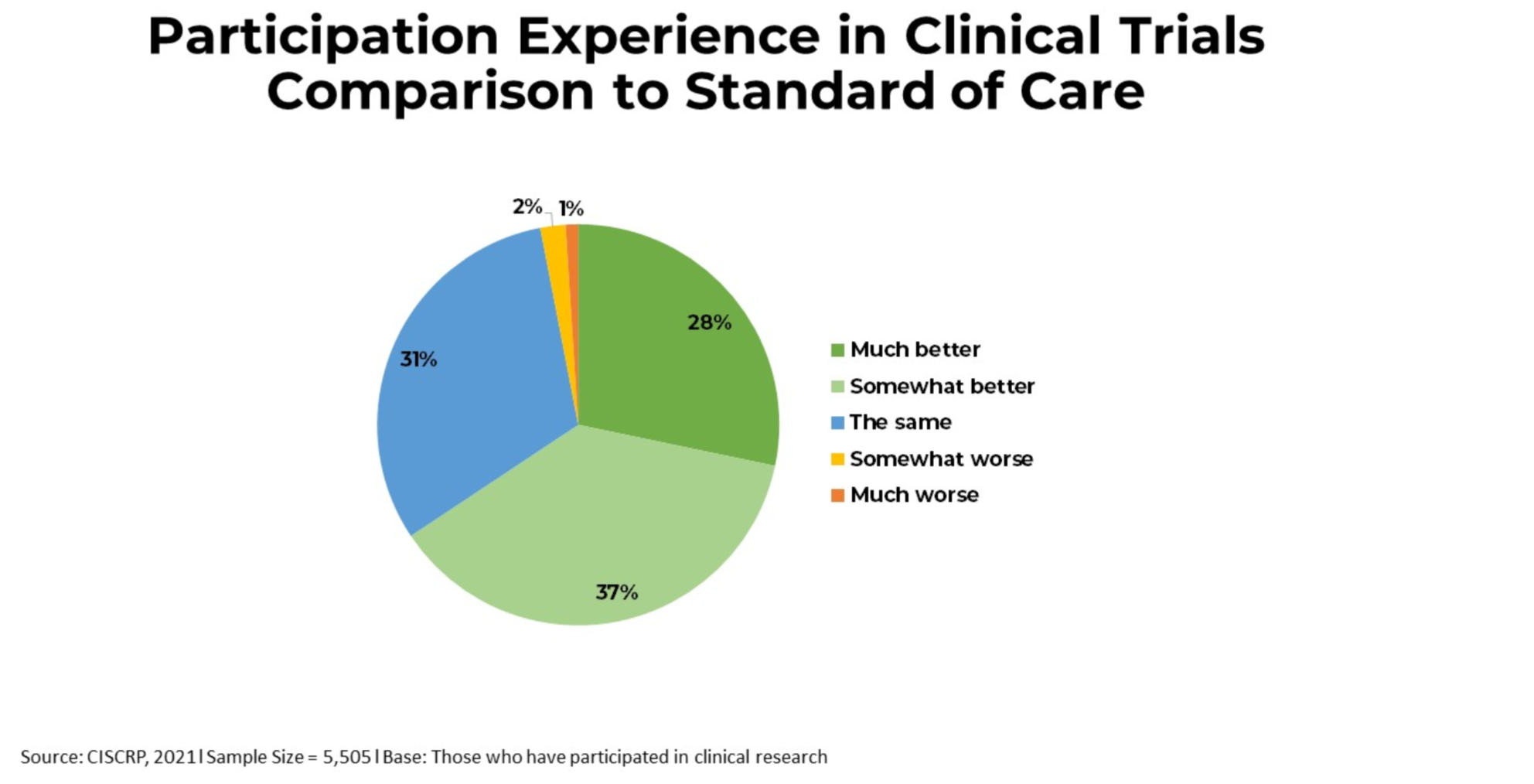
Eligibility Criteria and Selection
Clinical trials have eligibility criteria based on factors like age, sex, disease type, previous treatments, and overall health. Not everyone who applies will be accepted, as these criteria aim to reduce variability within the study group.
Finding Clinical Trials
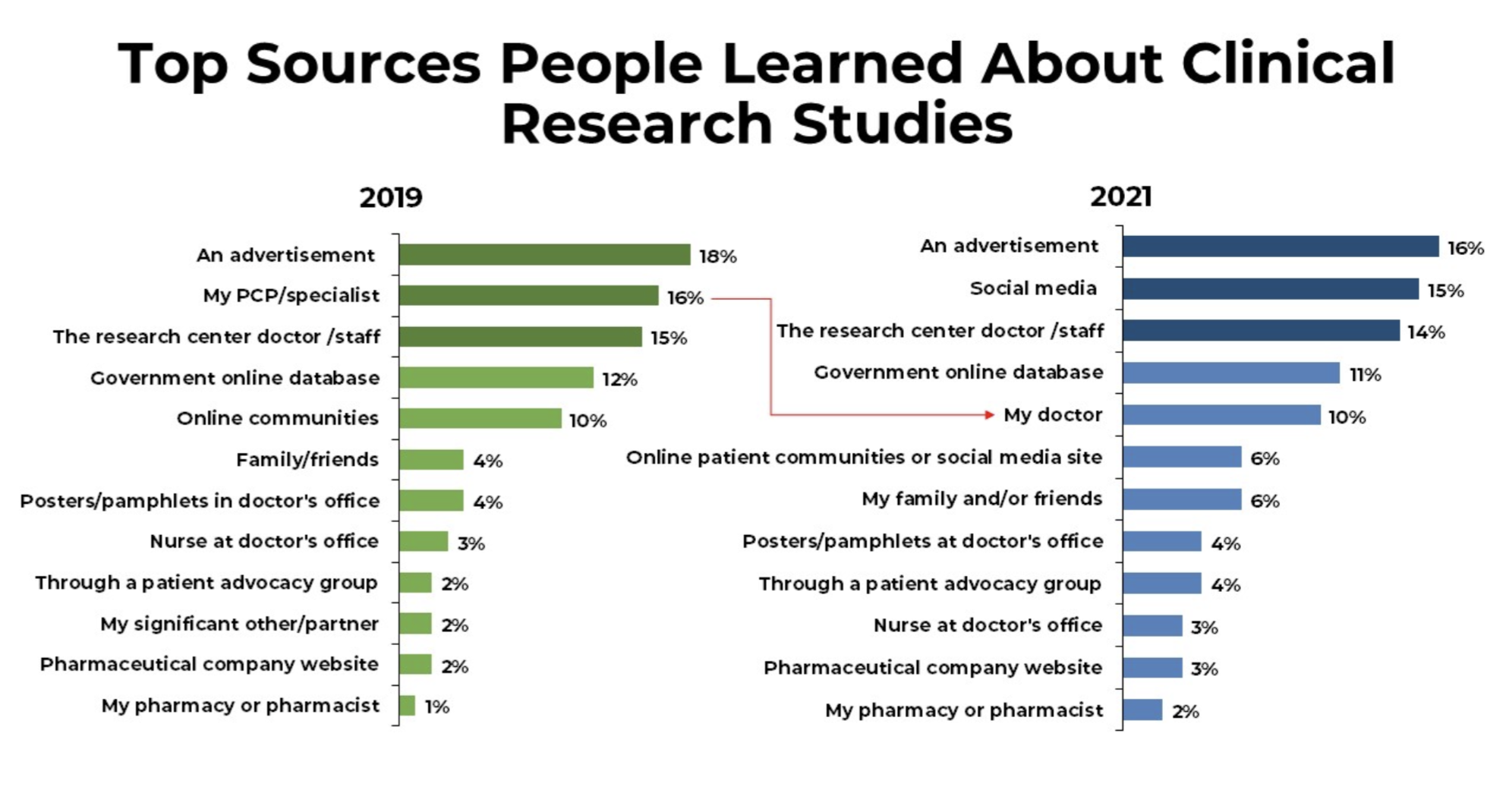
Clinical trials can be sponsored by pharmaceutical companies, federal agencies like the NIH, or individuals such as doctors. They are typically conducted at universities, medical centers, clinics, hospitals, and research sites. Or you can find a list of active clinical trials on our website simply by clicking the “Live Studies” button.
Ensuring Safety in Clinical Trials
Clinical trial participants’ safety is paramount, and rigorous regulations are in place to protect them. Participants receive complete and accurate information about the trial before signing an informed consent document. This document outlines their rights and the study’s details, including potential risks.
Questions to Ask Before Participating in a Clinical Trial
Before participating in a clinical trial, individuals should gather as much information as possible. Key questions to consider include:
- Purpose of the Study:
What is the main objective of this trial? - Study Duration and Procedures:
How long will the trial last, and what will be required of participants? - Placebo Use:
Does the study involve a placebo, and will my doctor know which treatment I receive? - Intervention Details:
How will the research intervention be administered? - Published Results:
What information is available about the research intervention’s results? - Costs and Insurance:
Do I have to pay for any part of the study, and will my insurance cover these expenses? - Travel and Childcare:
Does the study cover travel costs or childcare expenses? - Continuity of Care:
Can I continue using the treatment if it proves effective after the study? - Follow-Up Care:
Will I receive follow-up care after the study concludes? - Research Team:
How experienced are the physician and study staff? - Safety Measures:
What measures are in place to ensure my safety? - Results Disclosure:
When and how will I receive the results of the clinical trial? - Placebo Disclosure:
Will I be informed if I receive a placebo?
- Benefits and risks:
Weigh the potential advantages and disadvantages of joining the trial. Assess whether the benefits align with your health goals. - Informed consent:
Make sure you receive complete and accurate information about the study, including potential risks, and that you sign an informed consent document acknowledging your understanding and the voluntary nature of your participation.
Ethical Considerations
Participating in a clinical trial is a personal choice that should be made voluntarily and without external pressure. Informed consent is a fundamental aspect of clinical research, ensuring that participants understand and agree to the study’s terms. Participants are free to withdraw from a trial at any time.
Enrolling in Clinical Trials
It’s essential to find clinical trials that match your needs and interests. You can start by asking your healthcare provider for recommendations. Additionally, you can explore resources such as the FDA Clinical Trials Search, Clinicaltrials.gov, the National Cancer Institute, and AIDS Clinical Trials and Information Services to find relevant trials.
Participate in Clinical Trials for Money
While some clinical trials offer compensation for participation, it’s important to prioritize your health and align your motivations with the broader goals of advancing medical science. Monetary incentives should not be the sole reason for participating.
Benefits of Participating in Clinical Trials
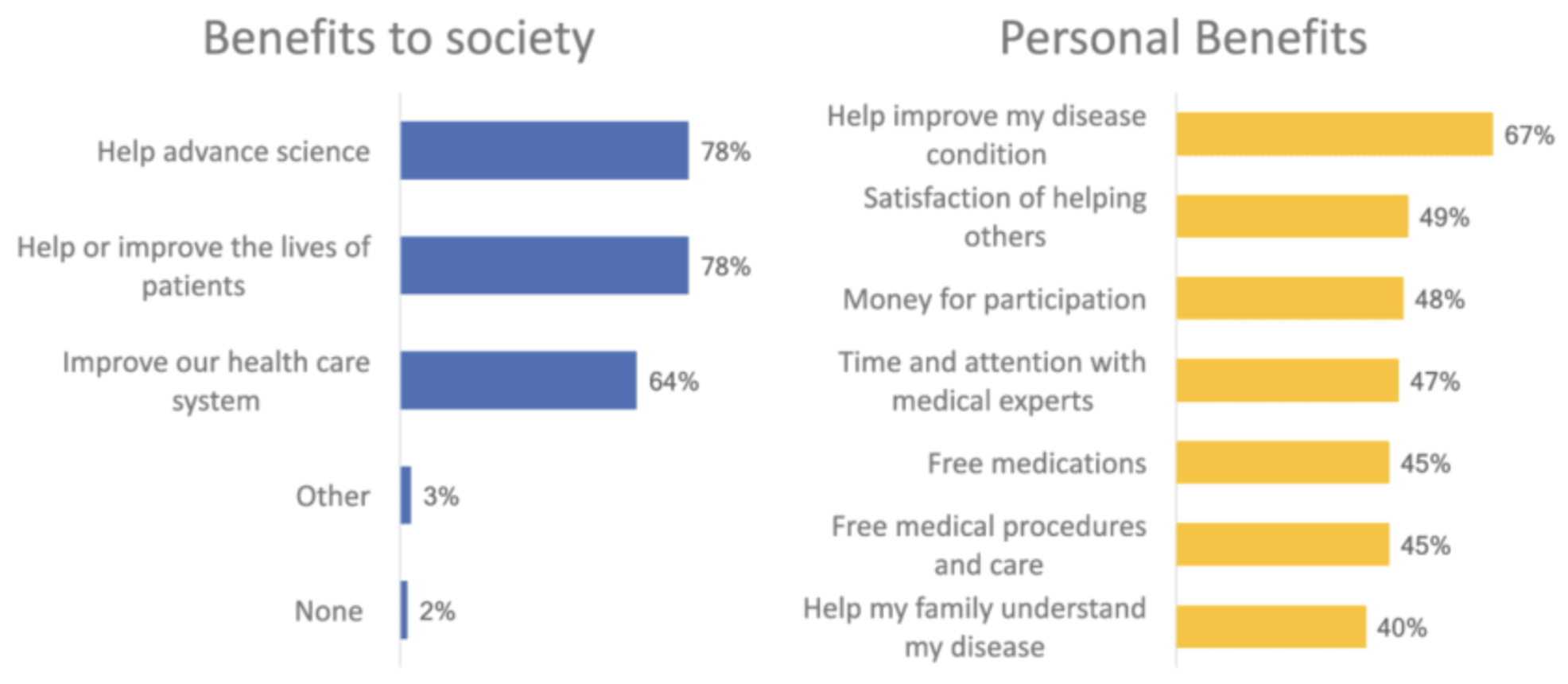
Participating in clinical trials can have various benefits, including access to cutting-edge treatments, expert medical care, and the opportunity to contribute to medical advancements that benefit society as a whole.
Tips on how to participate in clinical trials in 2024

- Do Your Homework:
Research the clinical trial thoroughly. Understand its purpose, procedures, potential risks, and benefits before making a decision. - Ask Questions:
Don’t hesitate to ask the research team any questions or concerns you have about the trial. It’s crucial to have a clear understanding. - Consult Your Healthcare Provider:
Discuss the clinical trial with your regular doctor to ensure it aligns with your current treatment plan and health goals. - Read the Informed Consent Carefully:
Take your time to read and understand the informed consent document. This document outlines your rights and the trial’s details. - Consider the Ethical Aspect:
Reflect on your motivation for participating in the trial. Ensure your decision aligns with your values and is made voluntarily. - Keep a Journal:
Maintain a journal to record your experiences and any changes in your health during the trial. This can be helpful for both you and the research team. - Follow Instructions:
Adhere to the trial’s protocols and follow all instructions provided by the research team. This ensures accurate data collection. - Report Adverse Events:
If you experience any adverse effects or discomfort during the trial, promptly inform the research team. Your well-being is their priority. - Stay Committed:
If you decide to participate, commit to the trial’s duration and requirements. Your contribution is essential for meaningful results. - Share Your Experience:
After the trial, consider sharing your experience with others. Your insights can help prospective participants make informed decisions about clinical trial participation.
Conclusion
Clinical trials are essential for advancing medical knowledge and improving healthcare. By participating in these trials, individuals can contribute to groundbreaking research, potentially benefit from innovative treatments, and even receive compensation for their involvement.
To make an informed decision about joining a clinical trial, it’s crucial to ask questions, consider the ethical aspects, and ensure that your participation aligns with your goals and values. Clinical research not only benefits individuals but also contributes to the betterment of healthcare for future generations.




